Molecular Weight of Air
These materials are only slightly hazardous to health and only breathing protection is needed. However there are still significant gaps in our understanding of these systems and challenges.
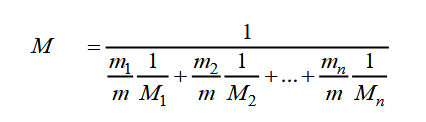
Quick Way To Find Molar Mass Of Air
For example water has a.

. Since air is made up predominantly of nitrogen gas the molecular weight of air is very close to that of nitrogen air g 2897 mol M. The gas constant for water vapor is. Low molecular weight gels are formed by the self-assembly of small molecules into anisotropic structures that form a network capable of immobilizing the solvent.
For example the molecular weight of nitrogen is Mnitrogen 140067 gmol. Similarly there were no effects of genotype or sex on grip strength at 18 months. 1E weight normalized grip strength deteriorated with increasing age in both Ubtf mice and their Ubtf littermates.
Thus Mgaseous nitrogen 280134 gmol. Temperature - Specific heat of Benzene Gas - C6H6 - at temperatures ranging 250 - 900 K. Benzene Gas - Specific Heat vs.
According to Merriam-Webster and the Online Etymology Dictionary the word molecule derives from the Latin moles or small unit of massThe word is derived from French molécule 1678 from New Latin molecula diminutive of Latin moles mass barrier. F n are their masses relative to the total mass of the mixture. A dose finding study.
Nitrogen in its gaseous or vapor state occurs as a diatomic molecule N2. Materials that on exposure would cause significant irritation but only minor residual injury including those requiring the use of an approved air-purifying respirator. Andrew M Massicotte P and Brooker LA.
Molecule a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance. Molecular masses are calculated from the atomic masses of each nuclide present in the molecule while relative molecular masses are calculated from the standard atomic weights of each elementThe standard atomic weight takes into account the isotopic distribution of the element in a given sample usually assumed to be normal. Low molecular weight heparin therapy in pediatric patients.
The word which until the late 18th century was used only in Latin form became popular after being used. BMI and weight gain have been recognized as an important risk factor for breast cancer said Angel referencing a study that found that an 11-pound increase in BMI corresponded to a 2 increase in. However there was a significant effect of age on weight normalized grip strength F 2471780 9799 P 00001.
For a mixture the molecular weight is a weighted mean of the molecular weights of the components. R d 287 J K-1 kg-1. Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component.
As seen in Fig. The gas constant for dry air is. Where m 1 m n are the molecular weights of the n gases.
Such gels are common with a huge number of different examples existing and they have many applications. The division of a sample of a substance into progressively smaller parts produces no change in either its composition or its chemical properties until parts consisting of single. Low molecular weight heparin in pediatric patients with thrombotic disease.
Massicotte P Adams M Marzinotto V Brooker LA and Andrew M.

2 2 Molecular Weight Determination Chemistry Libretexts
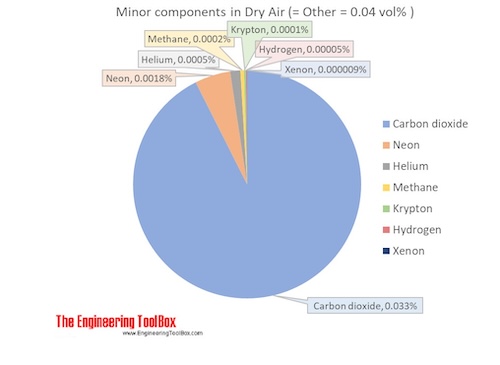
Air Composition And Molecular Weight
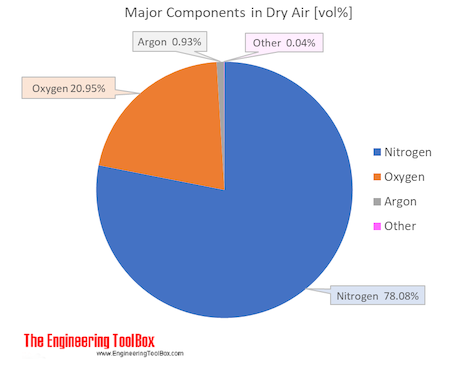
Air Composition And Molecular Weight

Average Molecular Weight Calculation Youtube
0 Response to "Molecular Weight of Air"
Post a Comment